Autism spectrum disorder (ASD) is a physical condition that affects children at an alarmingly high rate. One in 110 children will be diagnosed with autism. Abnormal social interaction, language difficulties and repetitive actions are all characteristics of autistic behavior. Autism also impairs a child’s ability to learn.
Exactly why autism occurs in one child and not another is unknown to scientists, although a combination of genetics and environment may play a role. Scientific evidence suggests that autism is caused by a malfunction of the connections, or synapses, between brain cells.
Recently, Peter Penzes, Ph.D., an associate professor of physiology at Northwestern University determined that a molecule, mutations in which have been genetically associated with autism, is involved in synapse development and remodeling in nerve cells.
Dr. Penzes used his $40,000 BRF seed grant to further characterize the role of this molecule in mice. A strain of mice genetically engineered to be deficient in this molecule were to be used to determine how such a shortage affects behavior, as well as brain physiology. Experiments planned to show how the molecule signals and regulates synapse development and behavior in a functioning organism. The research also allowed to test predictions for how this molecule is associated with ASD. His research focused on the frontal cortex, an area of the brain typically associated with psychiatric disorders, including ASD.
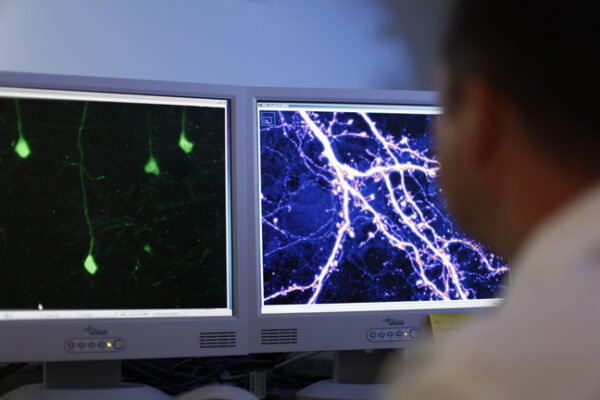
To study synapse development, Dr. Penzes used fluorescent microscopy to observe and measure nerve cells in mouse brain slices. These cells were to express a yellow fluorescent protein that was to allow for the visualization of the connections between nerve cells.
The behavioral portion of the study was to involve observing genetically altered mice that exhibit many of the social characteristics of autism without any unusual physical manifestations. Mice were to be tested for how they exhibit core behaviors such as social interaction, nesting and juvenile play, as well as how they communicate. A variety of maze tests were to assess learning capacity and response to change.
Insights from Dr. Penzes’ research help scientists understand the electrical connections in brain cells that may be disrupted in autism cases. Understanding biological mechanisms that trigger autism can lead to the identification of potential targets for therapy. Targeting this molecule may be fruitful because it has a high likelihood of oral bioavailability and low toxicity in humans.
The knowledge realized through Dr. Penzes’ research allows a better understanding of other neurodevelopmental disorders. Defects similar to the alterations in synapses prevalent in autism are also present in mental retardation, fragile-X syndrome and Down syndrome.
Using the data collected from his 2011 Seed Grant, Dr. Peter Penzes was able to turn this $40,000 grant into over $3 million in additional funding from the National Institute of Mental Health.